According to a study just published in the journal Energy Storage Materials, a new battery design could make it easier to integrate renewable energy into the national grid at a lower cost, using abundant metals in the world. Earth. A research team led by the Department of Energy’s Pacific Northwest National Laboratory has demonstrated that a new design for a low-cost sodium metal and aluminum mesh energy storage battery provides a roadmap towards a safer and more scalable fixed energy storage system.
“We have demonstrated that this new molten salt battery design is capable of much faster charging and discharging than other conventional high-temperature sodium batteries,” said Guosheng Li, a materials scientist. operates at lower temperatures and maintains excellent energy storage.” at PNNL and the study’s principal investigator. “We are achieving similar performance with this new sodium-based chemical at temperatures 100 °C [212 °F] lower than currently commercially available high-temperature sodium battery technologies, while using using more abundant materials on Earth.”
1. Increased energy storage
Imre Gyuk, Director of the Office of Electricity, DOE Energy Storage Program, which supported this research, notes: “This battery technology, is made with locally available materials at a cost low, bringing us one step closer to meeting the nation’s clean energy goals.”
The new sodium-based molten salt battery uses two separate reactions. The team previously reported on a neutral molten salt reaction. The new discovery shows that this neutral molten salt can undergo further reaction into an acidic molten salt. It is important that this second acid reaction mechanism increases the capacity of the battery. Specifically, after 345 charge/discharge cycles at high current, this acid reaction mechanism retained 82.8% of the peak charging capacity.
The energy a battery can deliver during discharge is called its specific energy density, expressed in “watt hours per kilogram” (Wh/kg). Although the battery is in the experimental stage or “coin battery,” the researchers speculate that it could produce actual energy densities of up to 100 Wh/kg. For comparison, the energy density of lithium-ion batteries used in commercial electronics and electric vehicles is around 170-250 Wh/kg. However, the new sodium-aluminum battery design has the advantage of being cheap and easy to manufacture in the United States from much more abundant materials.
“With optimization, we hope that the specific energy density and life cycle can be reached even higher and longer,” added Li.

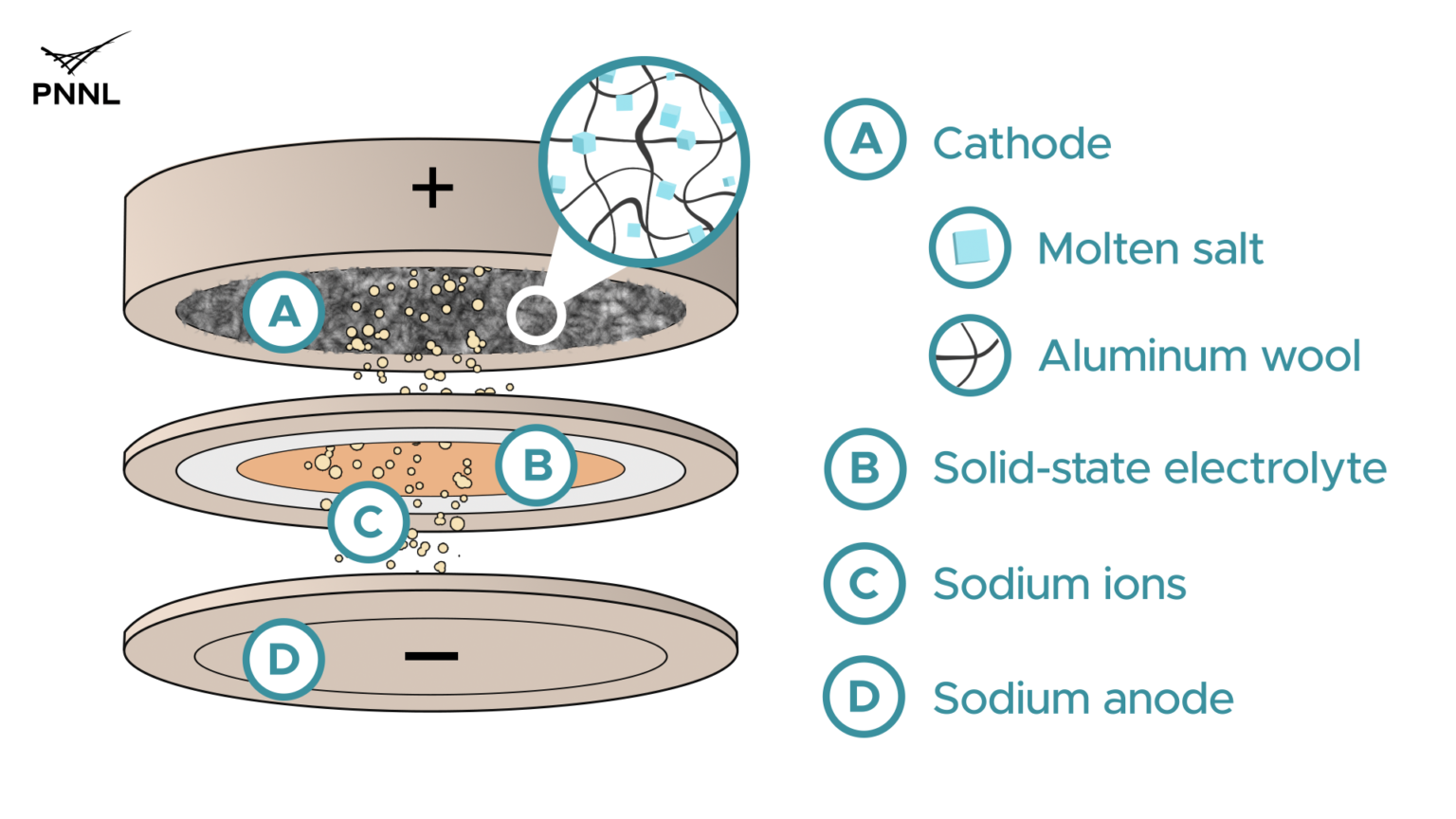
2. Sodium battery shows its capabilities
Indeed, the PNNL scientists teamed up with colleagues at US-based renewable energy pioneer Neexceris to assemble and test the batteries. Neexceris, through its new business Adena Power, supplied their patented solid-state, sodium-based electrolyte to PNNL for battery performance testing. This important battery component allows sodium ions to move from the cathode (anode) to the anode (cathode) of the battery when charging.
“Our main goal with this technology is to enable low-cost, everyday solar energy transfer to the grid,” said Vince Sprenkle, PNNL battery technology expert with over 30 patented designs. electricity for a period of 10 to 24 hours”. energy storage systems and related technologies. “This is a great point where we can start thinking about integrating higher levels of renewable energy into the grid to provide true grid resilience from renewable sources like wind and electricity. sun.”
Sprenkle is part of the team that developed the new flexible design of this battery, which also converts the battery from a traditional tube to a flat, expandable form that can be easily stacked and expanded. more as technology evolves from coin-sized batteries to larger ones. grid-scale display size. More importantly, this flat cell design allows for increased cell capacity by simply using a thicker cathode, which the researchers used in this study to demonstrate a folding capacity cell. three times with a continuous discharge time of 28.2 hours under laboratory conditions.
Most current battery technologies, including lithium-ion batteries, are well suited for short-term energy storage. To meet the demand for more than 10 hours of energy storage it will be necessary to develop new, low-cost, safe and long-lasting battery concepts that go beyond modern battery technologies. This study provides a promising laboratory-scale demonstration towards that goal.